Climate change has been a phenomenon known for its destructive ability for future generations of our planet. It refers to changes in global temperature and weather patterns occurring over long periods of time. Although these shifts can be natural, human activities have been the main cause of climate change predominantly due to the burning of fossil fuels that produce heat-trapping gasses. Ever since the beginning of the Industrial Revolution, there has been an increase in the burning of fossil fuels such as oil, coal, and natural gas for heat, transportation, and electricity. These shifts in the needs of humans have created an increase in concentrations of greenhouse gasses such as carbon dioxide, methane, and nitrous oxide that are being released into the atmosphere. These increased greenhouse gas emissions have sped a phenomenon known as the Greenhouse Effect, thus causing the Earth’s surface temperature to rise.

To reduce the spread of climate change further, researchers and scientists around the world are working on solutions to stop and reduce the effects of human modifications on the environment. Some of these solutions include a transition toward cleaner renewable sources of energy such as solar and wind power. Switches to sustainable transportation such as carpooling and biking have also made an appearance in the daily lives of many Americans in an effort to reduce greenhouse-gas emissions into the atmosphere. Today, many are taking an active role in society to advocate for a cleaner environment and a healthier Earth for future generations.
There are developments such as lithium-ion batteries that have helped mitigate the spread of climate change. There has been a recent breakthrough in scientific discovery from a team of researchers from the Department of Energy’s Pacific Northwest National Laboratory. They announced the creation of a low-cost, molten salt battery that can store energy for months at a time. If scaled up and spread amongst the general public, it may be able to accelerate the transition to a green electric grid.
For 50 years, these batteries have been explored in various forms as a solution for renewable energy due to their common material makeup and low cost. This older technology, though it has been the gold standard of energy storage for many years, however, has some downsides and limitations in modern industries. Even with the latest improvements in batteries, bulk energy storage offers a few hours of power before needing to be recharged.
These new “hibernating” batteries solved problems found in older models of lithium batteries. It is able to be charged with renewable energy seasonally for long periods of time. Rather than having a self-charge function during an idle period or having energy discharged into the environment as waste, the electricity is stored without a loss for several months.
This temperature-based hibernating battery consists of a metal anode (aluminum, magnesium, titanium), metal cathode (nickel, zinc, iron, and copper), molten salt electrode, and porous separator. The metal cathode and anode are sandwiched in a glass fiber separator. The molten salt electrolyte must be in a liquid state and operating at an extreme temperature for the battery to function.
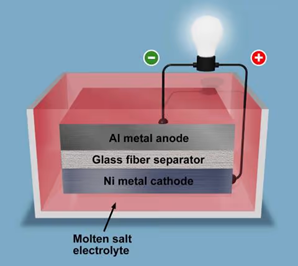
To prevent self-discharge, which would “freeze” the electrolytes when the battery is not in use, it is kept at room temperature. During the charging process, the temperature of the battery is raised by an external heat source, such as a heating cartridge to bring the electrolytes to a liquid state and start ion mobility in the battery. The glass fiber separator assists in insulating the cathode from the anode and reducing thermal expansion during solidification. As the concoction cools, the molten electrolyte solidifies into salts once the battery is not being used or charged. Through this process, the hibernating battery is able to store electricity efficiently.
When the battery is fully charged, it is cooled until it reaches room temperature, allowing the electrolyte to solidify and maintain the battery’s state. Thus, it is also referred to as a “freeze-thaw battery.” Self-discharge of the battery can be stopped at room temperature because the solidified electrolyte lacks ion transport capabilities. When a battery reaches its ideal operating temperature, it discharges, supplying power to the grid.
The researchers are hopeful that the technology will eventually enable a type of seasonal energy storage in which energy is captured at one point in the year and used at another. Additionally, because the battery can remain dormant while still retaining the majority of its stored energy, it would only require charging and discharging a few times per year. New technologies developed by scientists like the hibernating battery are paving the way for a lessened spread of climate change and creating a better tomorrow for future generations on Earth.